
S tructure
Alcohols, Phenols, and Thiols
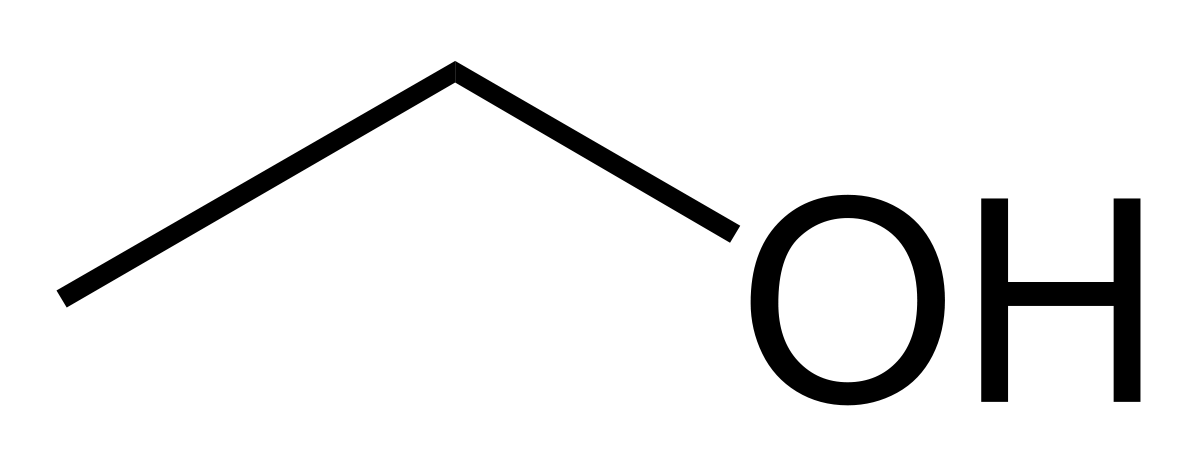
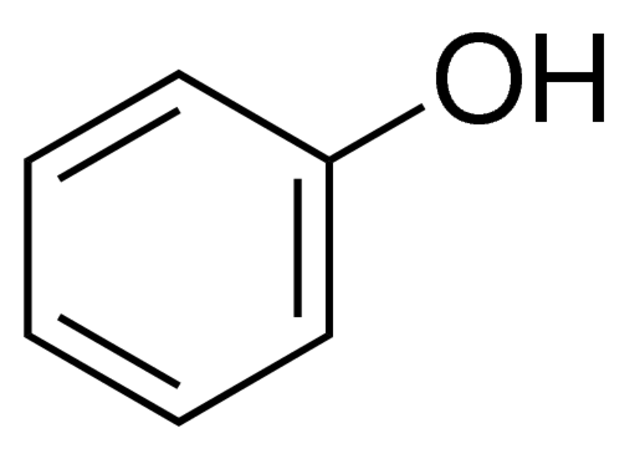
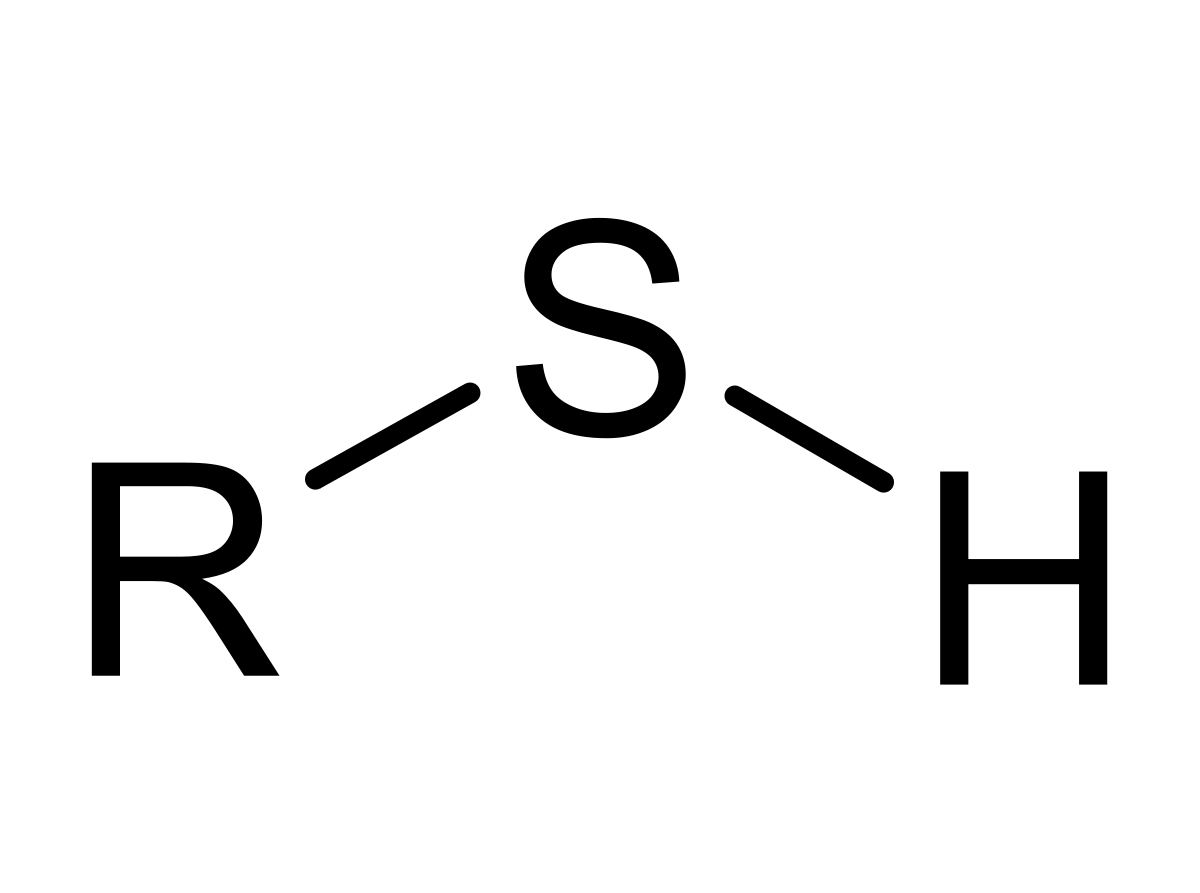
Alcohols, phenols, and thiols are all represented with their represctive primary functional side groups (-OH [hydroxyl] for alcohols/phenols and -SH for thiols) connecting off the main functional group/chain of the molecule. Alchohols have -OH groups coming off of the main carbon chain of a molecule at any carbon location. Phenols have the same structure, except that the main carbon chain is replaced with a aromatic molecule instead. Thiols also follow the structure of alcohols, except that the -OH side group(s) is replaced with an -SH. (In the example, R stands for a carbon chain). For alcohols, they are classified by being considered a primary (1o), secondary (2o), or tertiary (3o) alcohol. These are designated by the amount of the carbons bonded to the carbon containing the -OH group. For instance, if there is two carbons bonded to the carbon containing the -OH, it would be a secondary alcohol. (Three bonded carbons would be tertiary and one bonded carbon would be primary).
General Formula
The general formula for alcohols (for an alkane main carbon group):
CnH2n+1OH (R-OH)
The general formula for phenols:
AR-OH
The general formula for thiols:
R-SH
where n is all real numbers that are positive.

B ack