
N omenclature
The Process
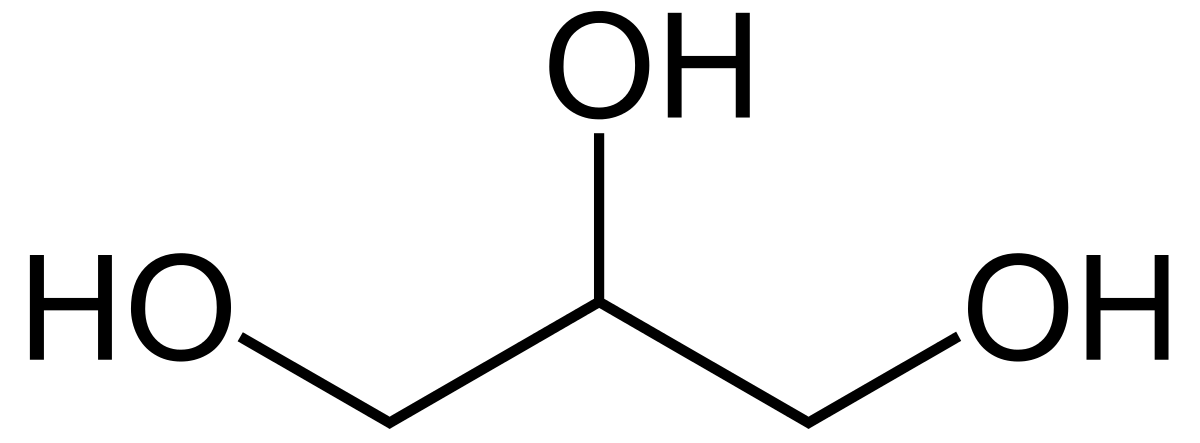
In general, alchohols, phenols, and thiols follow the same IUPAC rules discussed in the first alkanes portion of the portfolio. Each compounds/chemicals have unique suffix's. For alcohols the suffix is -ol (EX: 1-Propanol/Propan-1-ol), for phenols the suffix is -phenol (EX: 2,4-dinitrophenol, etc.), and for thiols its -thiol (EX: methanethiol). Alcohols, phenols, and thiols all have higher priority over double/triple bonds and carbon side/main chains in terms of IUPAC naming, meaning you want to number the order of the carbon atoms based off of which path results in the lowest carbon number for where the functional group (for an alcohol, thiol, etc.) is attached. Phenols also have a benzene attached to the -OH (as discussed in structure) resulting in the ortho-meta-para naming still being used for the chemical, this is noted below. Unlike alcohols and thiols, numbering the location of the functional groups (-OH and -SH) for phenols is only necessary if two or more of the -OH groups are present, as it is assumed the -OH is the start of the numbering of carbons. For alcohols and phenols, the location of the -OH/-SH groups are mandatory for any amount.
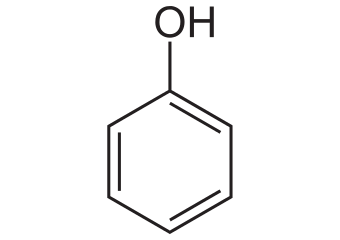
>Ortho (o) = 1,2 ringed side chains
>Meta (m) = 1,3 ringed side chains
>Para (p) = 1,4 ringed side chains
Alchohols
...-(# off the main chain)-(main chain carbon # prefix)(# of carbons in side chain prefix)yl(# of carbons in main chain prefix)-(# of carbon -OH location)-ol
...-2-methylpropan-2-ol (or tert-butanol)
Phenols
...-(# off the main chain)-(main chain carbon # prefix)(# of carbons in side chain prefix)yl(# of carbons in main chain prefix)-phenol
...-2-ethylphenol
Thiols
...-(# off the main chain)-(main chain carbon # prefix)(# of carbons in side chain prefix)(# of carbons in main chain prefix)-(# of carbon -SH location)-thiol
...-propane-2-thiol
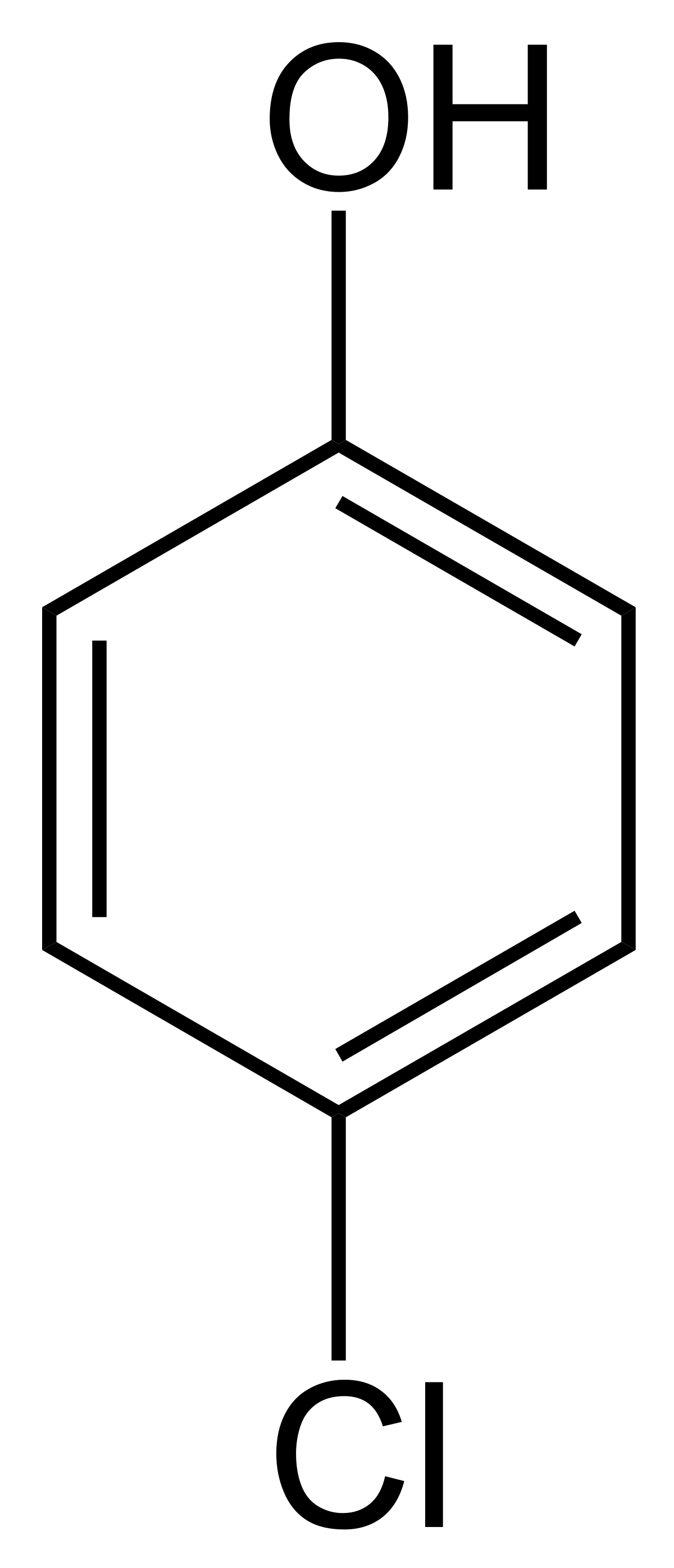 (p)-chlorophenol
(p)-chlorophenol
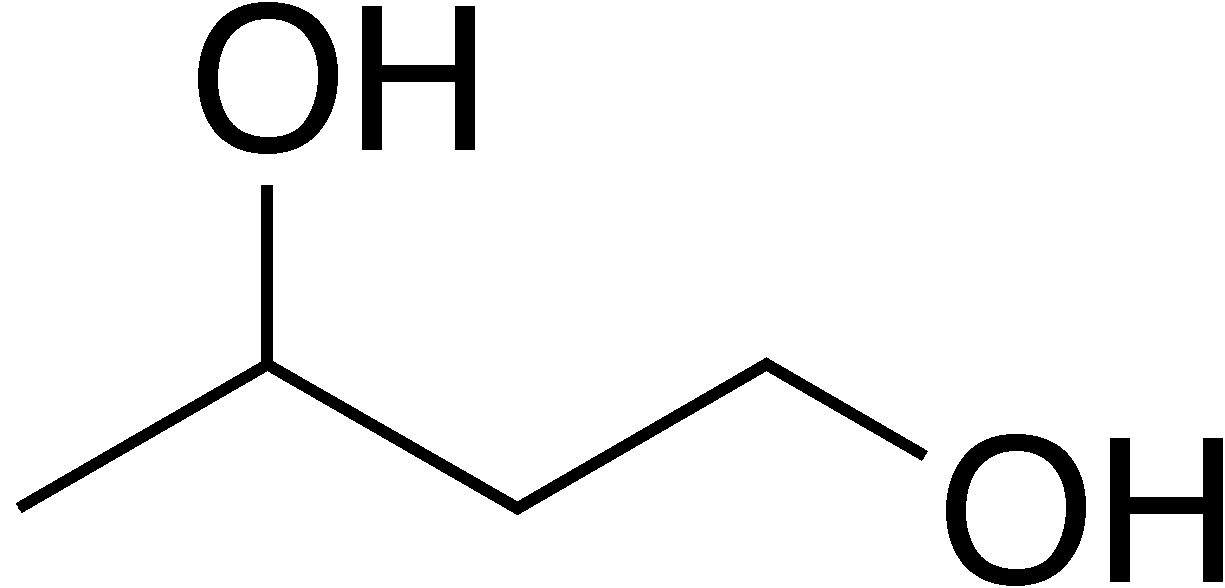 1,3-butandiol (the prefixes for multiple atoms/groups [di,tri,etc.] still applies to these molecules!)
1,3-butandiol (the prefixes for multiple atoms/groups [di,tri,etc.] still applies to these molecules!)
 4-methylcyclohexanethiol
4-methylcyclohexanethiol
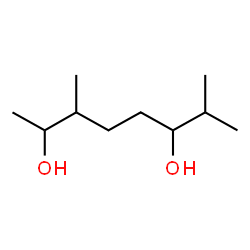 3,7-dimethyl-2,6-octanediol
3,7-dimethyl-2,6-octanediol

B ack