
N omenclature
The Process
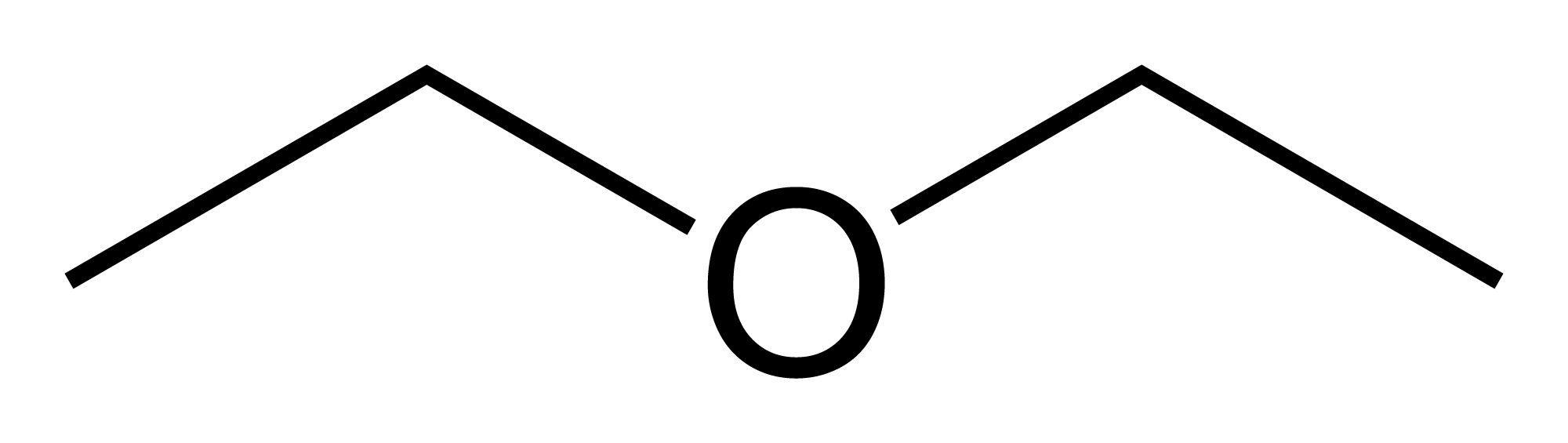
In general, ethers compounds follow the same IUPAC rules discussed in the first alkanes portion of the portfolio but with some differences. They are based on the IUPAC name of the longest chain alkane attached to the oxygen. The shorter chain is named as an alkoxy substituent (alkane naming with the -ane replaced by an -oxy, for example, propoxy). The number before the alkoxy name is the number off the largest chain of carbons where the oxygen is located (the oxygen that has the smaller carbon chain attached to it).
...-()(# of carbons in the smaller chain prefix)oxy(# of carbons in the largest carbon chain alkane naming)
...-2-ethoxyhexane
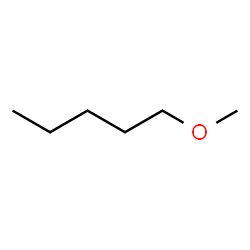 1-methoxypentane
1-methoxypentane
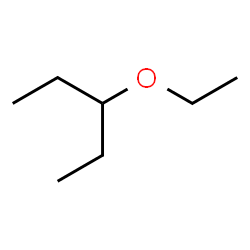 3-ethoxypentane
3-ethoxypentane
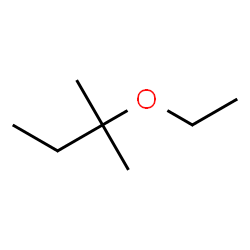 2-ethoxy-2-methylbutane (alphabetical order still!)
2-ethoxy-2-methylbutane (alphabetical order still!)
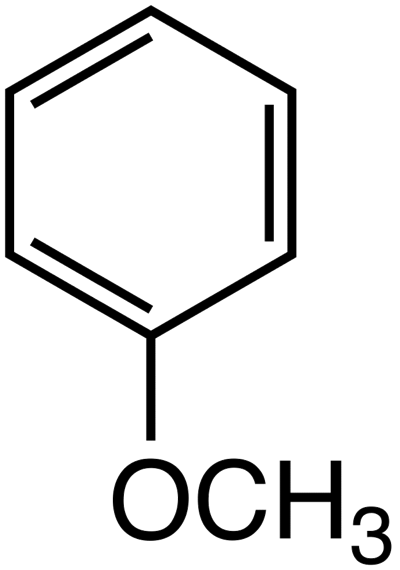 methoxybenzene (This is an aromatic ether!)
methoxybenzene (This is an aromatic ether!)

B ack